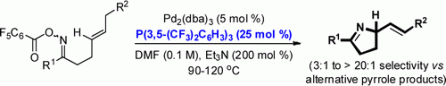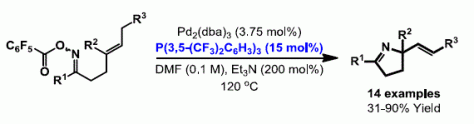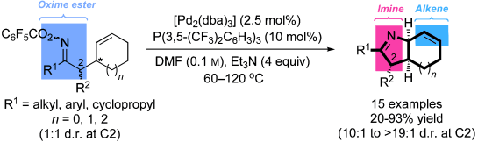103. R. L. de Carvalho, J. M. Wood, R. G. Almeida, N. G. Berry, E. N. da Silva Júnior* and J. F. Bower*; The synthesis and reactivity of naphthoquinonynes. Angew. Chem. Int. Ed. 2024, In press.
102. F. Hong, T. P. Aldhous, P. D. Kemmitt and J. F. Bower*; A directed enolization strategy enables byproduct free construction of contiguous stereocentres en route to complex amino acids. Nat. Chem. 2024, In press.
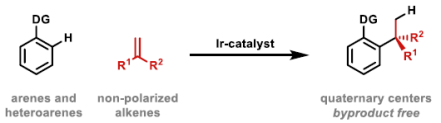
101. C. Jing, W. Mao and J. F. Bower*; Iridium-catalyzed enantioselective alkene hydroalkylation via a heteroaryl-directed enolization-decarboxylation sequence. J. Am. Chem. Soc. 2023, 145, 23918-23924.

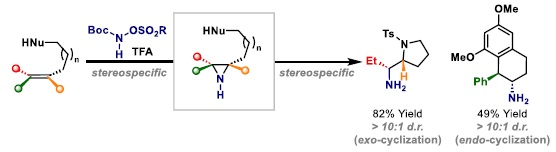
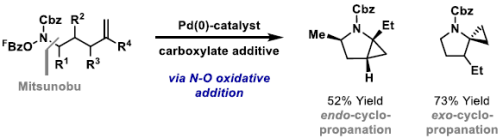
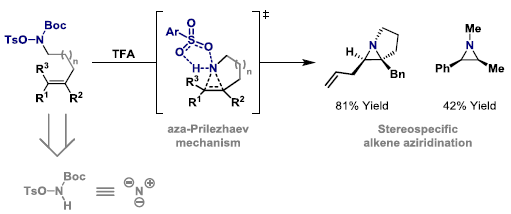
100. M. J. S. Smith, W. Tu, C. M. Robertson and J. F. Bower*; Stereospecific aminative cyclizations triggered by intermolecular aza-Prilezhaev alkene aziridination. Angew. Chem. Int. Ed. 2023, 62, e202312797. (Highlighted in Org. Process Res. Dev. DOI: 10.1021/acs.oprd.3c00460)
99. S. A. Scott, J. A. Cadge, G. K. Boden, J. F. Bower* and C. A. Russell*; A hemilabile NHC-gold complex and its application to the redox neutral 1,2-oxyarylation of feedstock alkenes. Angew. Chem. Int. Ed. 2023, 62, e202301526.

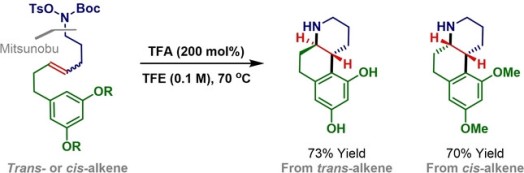
98. Y. Zhu, M. J. S. Smith, W. Tu and J. F. Bower*; A stereospecific alkene 1,2-aminofunctionalization platform for the assembly of complex nitrogen containing ring systems. Angew. Chem. Int. Ed. 2023, 62, e202301262.
97. R. J. Adams, P. G. Pringle* and J. F. Bower*; 1,3,5,7-Tetramethyl-8-phenyl-2,4,6-trioxa-8-phosphatricyclo[3.3.1.13,7]decane in e-EROS Encyclopedia of Reagents for Organic Synthesis, Wiley-VCH, 2023, In press.
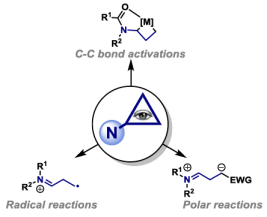
96. O. O. Sokolova, A. G. Dalling and J. F. Bower*; C-C bond activations of minimally activated cyclopropanes. Synlett 2023, 34, 1317-1326. Invited contribution to a special issue in honour of Professor Masahiro Murakami.
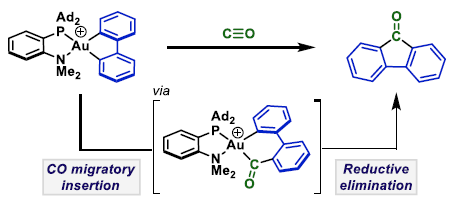
95. J. A. Cadge, P. J. Gates, J. F. Bower* and C. A. Russell*; Migratory insertion of CO into a Au-C bond. J. Am. Chem. Soc. 2022, 144, 19719-19725.
94. C. Jing, B. T. Jones, R. J. Adams and J. F. Bower*; Cyclopropane-fused N-heterocycles via aza-Heck triggered C(sp3)-H functionalization cascades. J. Am. Chem. Soc. 2022, 144, 16749-16754.
93. P. Cooper, A. G. Dalling, E. H. E. Farrar, T. P. Aldhous, S. Grélaud, E. Lester, L. J. Feron, P. D. Kemmitt, M. N. Grayson* and J. F. Bower*; Atom and step economical synthesis of acyclic quaternary centers via iridium-catalyzed hydroarylative cross-coupling of 1,1-disubstituted alkenes. Chem. Sci. 2022, 13, 11183-11189.
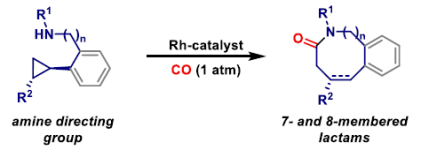
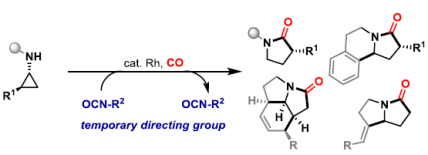
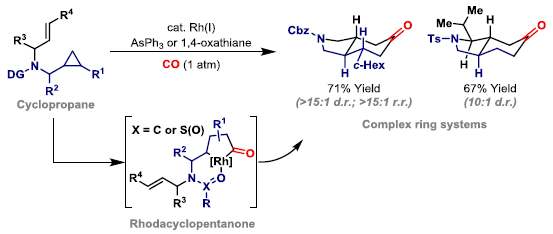
92. A. D. J. Calow, D. Dailler and J. F. Bower*; Carbonylative N-heterocyclization via nitrogen-directed C-C bond activation of non-activated cyclopropanes. J. Am. Chem. Soc. 2022, 144, 11069-11074.
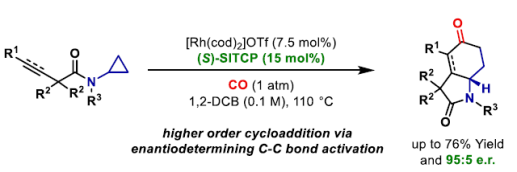
91. O. O. Sokolova and J. F. Bower*; An endo-directing group strategy unlocks enantioselective (3+1+2) carbonylative cycloadditions of aminocyclopropanes. Angew. Chem. Int. Ed. 2022, 61, e202205007.
90. G. A. M. Jardim, R. L. de Carvalho, M. P. Nunes, L. A. Machado, L. D. Almeida, K. A. Bahou, J. F. Bower* and E. N. da Silva Júnior*; Looking deep into C–H functionalization: the synthesis and application of cyclopentadienyl and related metal catalysts. Chem. Commun. 2022, 58, 3101-3121. Invited contribution to a thematic collection on the “Functionalization of unreactive C-H bonds”.
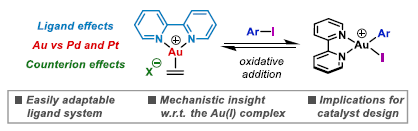
89. J. A. Cadge, J. F. Bower* and C. A. Russell*; A systematic study of the effects of complex structure on aryl iodide oxidative addition at bipyridyl-ligated Au(I) centers. Angew. Chem. Int. Ed. 2021, 60, 24976-24981. (open access)
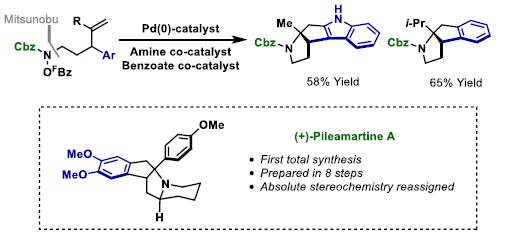
88. B. T. Jones, J. García-Cárceles, L. Caiger, I. R. Hazelden, R. J. Lewis, T. Langer and J. F. Bower*; Complex polyheterocycles and the stereochemical reassignment of pileamartine A via aza-Heck triggered aryl C-H functionalization cascades. J. Am. Chem. Soc. 2021, 143, 15593-15598. (open access)
87. C. Jing, J. J. Farndon and J. F. Bower*; Dearomatizing amination reactions. Chem. Rec. 2021, 21, 2909-2926. Invited contribution to the special issue for the 18th Brazilian Meeting on Organic Synthesis (BMOS).
86. L. G. O’Neil and J. F. Bower*; Electrophilic aminating agents in total synthesis. Angew. Chem. Int. Ed. 2021, 60, 25640-25666.
85. T. P. Aldhous, R. W. M. Chung, A. G. Dalling and J. F. Bower*; Enantioselective intermolecular Murai-type alkene hydroarylation reactions. Synthesis 2021, 53, 2961-2975. Invited contribution to a special issue in honour of Professor Shinji Murai.
84. O. O. Sokolova and J. F. Bower*; Selective carbon-carbon bond cleavage of cyclopropylamine derivatives. Chem. Rev. 2021, 121, 80-109. Invited contribution to the 2021 thematic issue on “Carbon-Carbon Bond Cleavage in Stereoselective Synthesis.”
83. L. Dantas-Pereira, E. F. Cunha-Junior, V. V. Andrade-Neto, J. F. Bower, G. A. M. Jardim, E. N. da Silva Júnior, E. C. Torres-Santos and R. F. S. Menna-Barreto*; Naphthoquinones and derivatives for chemotherapy: perspectives and limitations of their anti-trypanosomatids activities. Curr. Pharm. Des. 2021, 27, 1807-1824.
82. G.-W. Wang, O. O. Sokolova, T. A. Young, E. M. S. Christodoulou, C. P. Butts and J. F. Bower*; Carbonylative C-C bond activation of aminocyclopropanes using a temporary directing group strategy. J. Am. Chem. Soc. 2020, 142, 19006-19011.
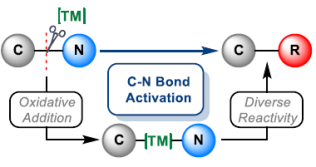
81. J. García-Cárceles, K. A. Bahou, and J. F. Bower*; Recent methodologies that exploit oxidative addition of C-N bonds to transition metals. ACS Catal. 2020, 10, 12738-12759.
80. J. M. Wood, N. S. Satam, R. G. Almeida, V. S. Cristani, D. P. Lima, L. D. Pereira, K. Salomão, R. F. S. Menna-Barreto, I. N. N. Namboothiri,* J. F. Bower* and E. N. da Silva Júnior*; Strategies towards potent trypanocidal drugs: application of Rh-catalyzed [2+2+2] cycloadditions, sulfonyl phthalide annulation and nitroalkene reactions for the synthesis of substituted quinones and their evaluation against Trypanosoma cruzi. Bioorg. Med. Chem. 2020, 28, 115565.
79. A. S. Henderson, J. F. Bower* and M. C. Galan*; Pseudo-enantiomeric carbohydrate-based N-heterocyclic carbenes as promising chiral ligands for enantiotopic discrimination. Org. Biomol. Chem. 2020, 18, 3012-3016. Invited contribution to the themed collection on “Glycosylation: new methodologies for oligosaccharide and glycoconjugate synthesis and their applications”.
78. J. A. Cadge, H. A. Sparkes, J. F. Bower* and C. A. Russell*; Oxidative addition of alkenyl and alkynyl iodides to a Au(I) complex. Angew. Chem. Int. Ed. 2020, 59, 6617-6621.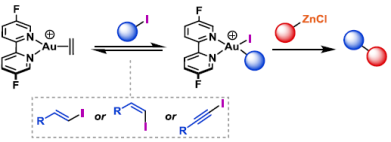
77. G.-W. Wang, O. Boyd, T. A. Young, S. M. Bertrand and J. F. Bower*; Rhodacyclopentanones as linchpins for the atom economical assembly of diverse polyheterocycles. J. Am. Chem. Soc. 2020, 142, 1740-1745.
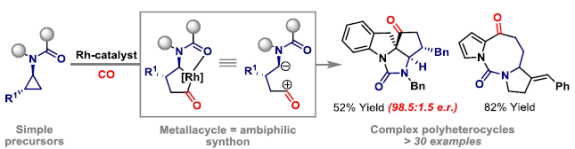
76. J. M. Wood, E. N. da Silva Júnior* and J. F. Bower*; Rh-catalyzed [2+2+2] cycloadditions with benzoquinones: de novo access to naphthoquinones for lignan and type II polyketide synthesis. Org. Lett. 2020, 22, 265-269.
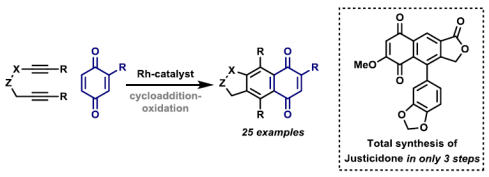
75. O. Boyd, G.-W. Wang, O. O. Sokolova, A. D. J. Calow, S. M. Bertrand and J. F. Bower*; Modular access to eight-membered N-heterocycles by directed carbonylative C-C bond activation of aminocyclopropanes. Angew. Chem. Int. Ed. 2019, 58, 18844-18848.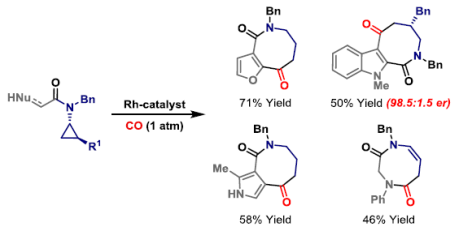
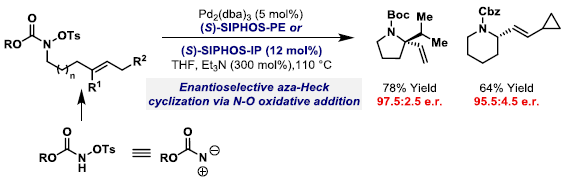
74. X. Ma, I. R. Hazelden, T. Langer, R. H. Munday and J. F. Bower*; Enantioselective aza-Heck cyclizations of N-(tosyloxy)carbamates: synthesis of pyrrolidines and piperidines. J. Am. Chem. Soc. 2019, 141, 3356-3360. (Highlighted in SYNFACTS 2019, 15, 483)
73. A. G. Dalling, T. Yamauchi, N. G. McCreanor, L. Cox and J. F. Bower*; Carbonylative C-C bond activation of electron-poor cyclopropanes: rhodium-catalyzed (3+1+2) cycloadditions of cyclopropylamides. Angew. Chem. Int. Ed. 2019, 58, 221-225.

72. A. D. J. Calow and J. F. Bower*; Rhodium(I)-catalyzed reductive carbon-carbon bond formation in Rhodium catalysis in organic synthesis: methods and reactions, K. Tanaka (Ed.), Wiley-VCH, 2019, pp 133-160. (book chapter)
71. J. J. Farndon, T. A. Young and J. F. Bower*; Stereospecific alkene aziridination using a bifunctional amino-reagent: an aza-Prilezhaev reaction. J. Am. Chem. Soc. 2018, 140, 17846-17850.
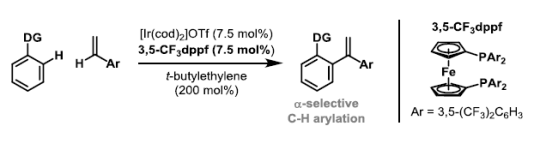
70. P. Cooper, G. E. M. Crisenza, L. J. Feron and J. F. Bower*; Iridium-catalyzed α-selective arylation of styrenes by dual C-H functionalization. Angew. Chem. Int. Ed. 2018, 57, 14198-14202.
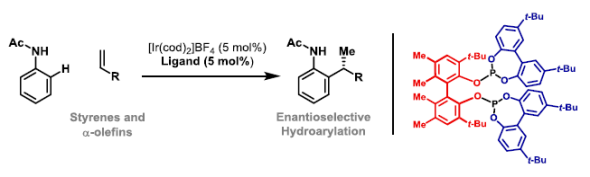
69. S. Grélaud, P. Cooper, L. J. Feron and J. F. Bower*; Branch-selective and enantioselective iridium-catalyzed alkene hydroarylation via anilide-directed C-H oxidative addition. J. Am. Chem. Soc. 2018, 140, 9351-9356. (Highlighted in SYNFACTS 2018, 14, 1165; Highlighted in Org. Process Res. Dev. 2019, 23, 2287−2301)
68. A. G. Dalling and J. F. Bower*; Synthesis of nitrogen heterocycles via directed carbonylative C-C bond activation of cyclopropanes. Chimia 2018, 72, 595-600. (review article)
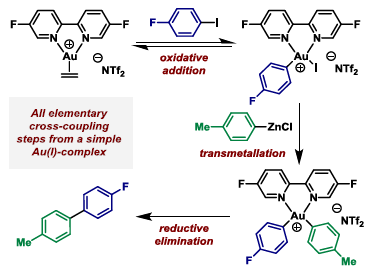
67. M. J. Harper, C. J. Arthur, J. Crosby, E. J. Emmett, R. L. Falconer, A. J. Fensham-Smith, P. J. Gates, T. Leman, J. E. McGrady,* J. F. Bower* and C. A. Russell*; Oxidative addition, transmetallation and reductive elimination at a 2,2′-bipyridyl ligated gold center. J. Am. Chem. Soc. 2018, 140, 2743-2747. (open access)
66. I. R. Hazelden, R. C. Carmona, T. Langer, P. G. Pringle and J. F. Bower*; Pyrrolidines and piperidines by ligand-enabled aza-Heck cyclizations and cascades of N-(pentafluorobenzoyloxy)carbamates. Angew. Chem. Int. Ed. 2018, 57, 5124-5128. (open access)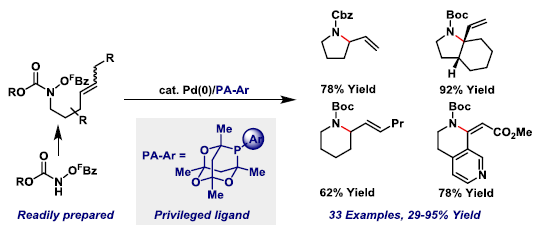
65. G.-W. Wang and J. F. Bower*; Modular access to azepines by directed carbonylative C-C bond activation of aminocyclopropanes. J. Am. Chem. Soc. 2018, 140, 2743-2747. (open access)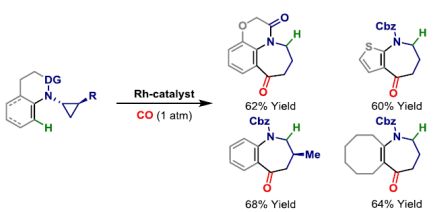
64. X. Ma, J. J. Farndon, T. Young, N. Fey and J. F. Bower*; A simple and broadly applicable C-N bond forming dearomatization protocol enabled by bifunctional amino reagents. Angew. Chem. Int. Ed. 2017, 56, 14531-14535. (open access; Highlighted in Org. Process Res. Dev. 2017, 21, 1873-1883)
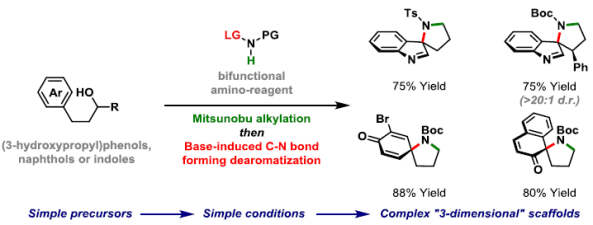
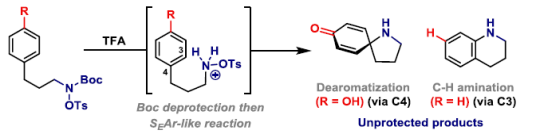
63. J. J. Farndon, X. Ma and J. F. Bower*; Transition metal free C-N bond forming dearomatizations and aryl C-H aminations by in situ release of a hydroxylamine-based aminating agent. J. Am. Chem. Soc. 2017, 139, 14005-14008. (open access)
62. M. J. Harper, E. J. Emmett, J. F. Bower* and C. A. Russell*; Oxidative 1,2-difunctionalization of ethylene via gold-catalyzed oxyarylation. J. Am. Chem. Soc. 2017, 139, 12386-12389. (open access)
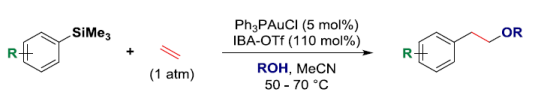
61. C. S. Buxton, D. C. Blakemore and J. F. Bower*; Reductive coupling of acrylates with ketones and ketimines by a nickel-catalyzed transfer hydrogenative strategy. Angew. Chem. Int. Ed. 2017, 56, 13824-13828. (open access; Highlighted in Org. Process Res. Dev. 2018, 22, 421-429; Highlighted in SYNFACTS 2018, 21)
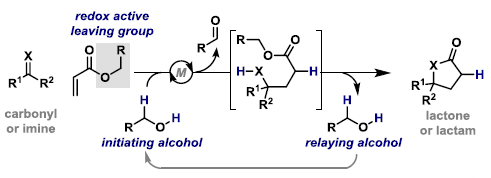
60. N. J. Race, I. R. Hazelden, A. Faulkner and J. F. Bower*; Recent developments in the use of aza-Heck cyclizations for the synthesis of chiral N-heterocycles. Chem. Sci. 2017, 8, 5248-5260. (open access)
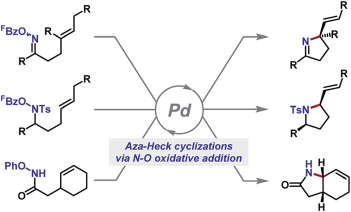
59. G. A. M. Jardim, T. L. da Silva, M. O. F. Goulart, C. A. de Simone, J. M. C. Barbosa, K. Salomão, S. L. de Castro, J. F. Bower and E. N. da Silva Júnior*; Rhodium-catalyzed C-H bond activation for the synthesis of quinonoid compounds: significant anti-Trypanosoma cruzi activities and electrochemical studies of functionalized quinones. Eur. J. Med. Chem. 2017, 136, 406-419.
58. N. J. Race, A. Faulkner, G. Fumagalli, T. Yamauchi, J. S. Scott, M. Rydén-Landergren, H. A. Sparkes and J. F. Bower*; Enantioselective Narasaka-Heck cyclizations: synthesis of tetrasubstituted nitrogen-bearing stereocenters. Chem. Sci. 2017, 8, 1981-1985. (open access; Highlighted in SYNFACTS 2017, 182; Highlighted in Org. Process Res. Dev. 2017, 21, 279-291).
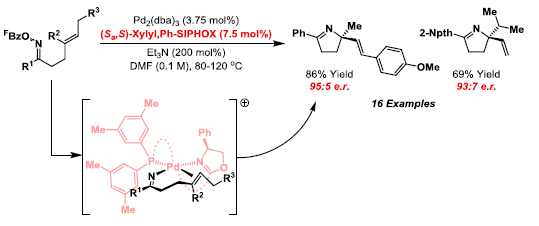
57. G. Fumagalli, S. Stanton and J. F. Bower*; Recent methodologies that exploit C-C single bond cleavage of strained ring systems by transition metal complexes. Chem. Rev. 2017, 117, 9404-9432. Invited contribution to the 2017 thematic issue on C-H and C-C functionalisation.
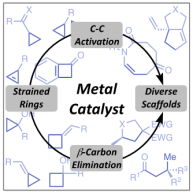
56. G.-W. Wang, N. G. McCreanor, M. H. Shaw, W. G. Whittingham and J. F. Bower*; New initiation modes for directed carbonylative C-C bond activation: rhodium-catalyzed (3+1+2) cycloadditions of aminomethylcyclopropanes. J. Am. Chem. Soc. 2016, 138, 13501-13504. (open access)
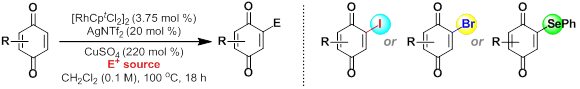
55. G. A. M. Jardim, J. F. Bower* and E. N. da Silva Júnior*; Rh-catalyzed reactions of 1,4-benzoquinones with electrophiles: C-H iodination, bromination and phenylselenation. Org. Lett. 2016, 18, 4454-4457.
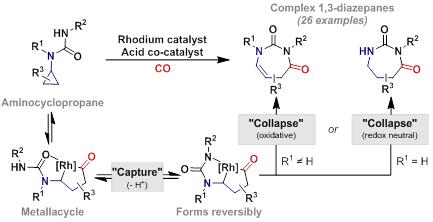
54. N. G. McCreanor, S. Stanton and J. F. Bower*; Capture-collapse heterocyclization: 1,3-diazepanes by C-N reductive elimination from rhodacyclopentanones. J. Am. Chem. Soc. 2016, 138, 11465-11468. (open access)
53. M. H. Shaw and J. F. Bower*; Synthesis and applications of rhodacyclopentanones derived from C-C bond activation. Chem. Commun. 2016, 52, 10817-10829 (open access review article)
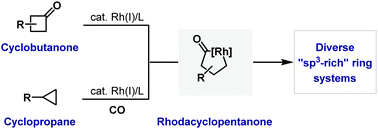
52. I. R. Hazelden, X. Ma, T. Langer and J. F. Bower*; Diverse N-heterocyclic ring systems via aza-Heck cyclizations of N-(pentafluorobenzoyloxy)sulfonamides. Angew. Chem. 2016, 128, 11364-11368; Angew. Chem. Int. Ed. 2016, 55, 11198-11202. (open access)
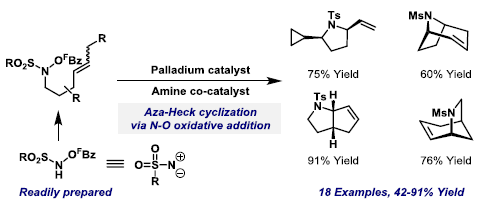
51. G. E. M. Crisenza, E. Dauncey and J. F. Bower*; C2-Alkenylation of N-heteroaromatic compounds via Brønsted acid catalysis. Org. Biomol. Chem. 2016, 14, 5820-5825. Invited contribution to the 2016 New Talent Special Issue. (open access)
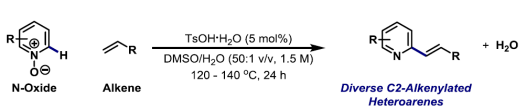
50. A. S. Henderson, J. F. Bower* and M. C. Galan*; Carbohydrates as enantioinduction components in stereoselective catalysis. Org. Biomol. Chem. 2016, 14, 4008-4017. (open access review article)
49. G. A. M. Jardim, E. N. da Silva Júnior* and J. F. Bower*; Overcoming naphthoquinone deactivation: rhodium-catalyzed C-5 selective C-H iodination as a gateway to functionalized derivatives. Chem. Sci. 2016, 7, 3780-3784. (open access)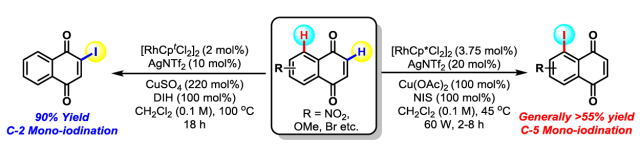
48. G. E. M. Crisenza and J. F. Bower*; Branch selective Murai-type alkene hydroarylation reactions. Chem. Lett. 2016, 45, 2-9. (open access review article)
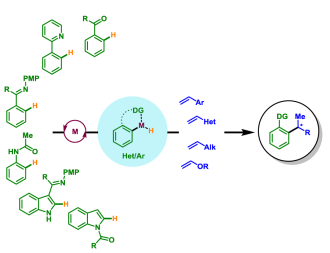

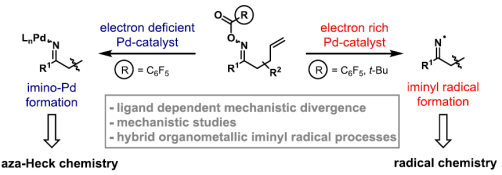
47. N. J. Race, A. Faulkner, M. H. Shaw and J. F. Bower*; Dichotomous mechanistic behavior in Narasaka-Heck cyclizations: electron rich Pd-catalysts generate iminyl radicals. Chem. Sci. 2016, 7, 1508-1513. (open access)
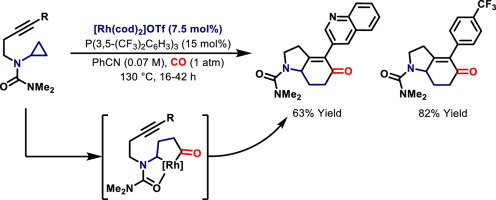
46. M. H. Shaw, W. G. Whittingham and J. F. Bower*; Directed carbonylative (3+1+2) cycloadditions of amino-substituted cyclopropanes and alkynes: reaction development and increased efficiencies using a cationic rhodium system. Tetrahedron 2016, 72, Symposium-in-print, Catalytic C–C bond formation by C–H functionalization and C–C bond cleavage, 2731-2741. (open access)
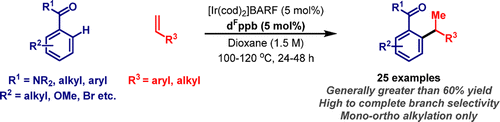
45. G. E. M. Crisenza, O. O. Sokolova and J. F. Bower*; Branch selective alkene hydroarylation by cooperative destabilization: iridium-catalyzed ortho-alkylation of acetanilides. Angew. Chem. 2015, 127, 15079-15083; Angew. Chem. Int. Ed. 2015, 54, 14866-14870. (open access)
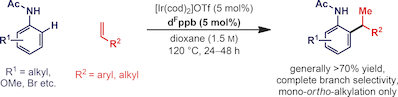
44. A. S. Henderson, S. Medina, J. F. Bower* and M. C. Galan*; Nucleophilic aromatic substitution as an approach to challenging carbohydrate-aryl ethers. Org. Lett. 2015, 17, 4846-4849. (open access)

43. M. H. Shaw, R. A. Croft, W. G. Whittingham and J. F. Bower*; Modular access to substituted azocanes via a rhodium-catalyzed cycloaddition-fragmentation strategy. J. Am. Chem. Soc. 2015, 137, 8054-8057. (open access)
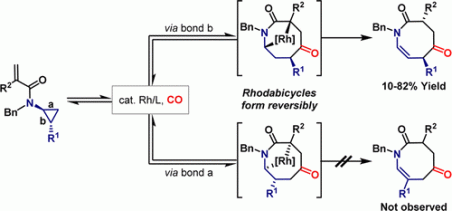
42. A. Faulkner, J. S. Scott and J. F. Bower*; An umpolung approach to alkene carboamination: palladium catalyzed 1,2-amino-acylation, -carboxylation, ‑arylation, -vinylation and -alkynylation. J. Am. Chem. Soc. 2015, 137, 7224-7230. (open access)
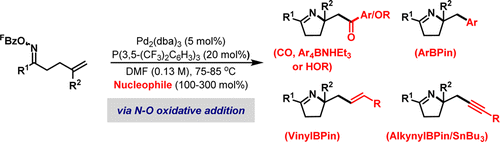
41. S. Medina, A. S. Henderson, J. F. Bower and M. C. Galan*; Stereoselective synthesis of glycosides using (salen)Co catalysts as promoters. Chem. Commun. 2015, 51, 8939-8941. (open access)
40. N. J. Race and J. F. Bower*; Synthesis of heteroaromatic compounds by alkene and enyne metathesis. Top. Heterocycl. Chem. 2017, 47, 1-32. (review article)
39. M. H. Shaw, N. G. McCreanor, W. G. Whittingham and J. F. Bower*; Reversible C-C bond activation enables stereocontrol in Rh-catalyzed carbonylative cycloadditions of aminocyclopropanes. J. Am. Chem. Soc. 2015, 137, 463-468. (open access)
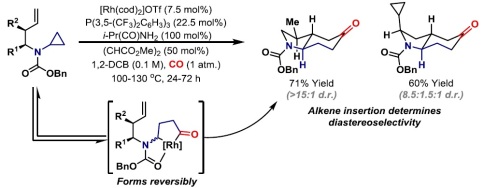
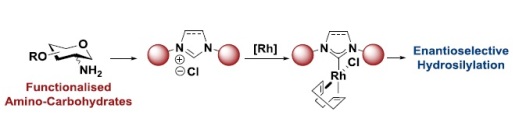
38. A. S. Henderson, J. F. Bower* and M. C. Galan*; Carbohydrate-based N-heterocyclic carbenes for enantioselective catalysis. Org. Biomol. Chem. 2014, 12, 9180-9183. (open access)
37. G. E. M. Crisenza, N. G. McCreanor and J. F. Bower*; Branch selective iridium-catalyzed hydroarylation of monosubstituted alkenes via a cooperative destabilization strategy. J. Am. Chem. Soc. 2014, 136, 10258-10261. (open access)
36. A. Faulkner, N. J. Race, J. S. Scott and J. F. Bower*; Copper catalyzed Heck-like cyclizations of oxime esters. Chem. Sci. 2014, 5, 2416-2421. (open access)
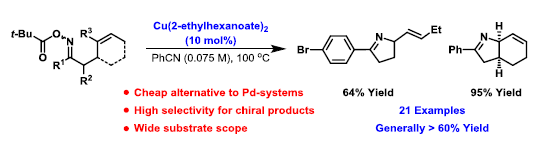
35. N. J. Race and J. F. Bower*; Palladium catalyzed cyclizations of oxime esters with 1,2-disubstituted alkenes: synthesis of dihydropyrroles. Org. Lett. 2013, 15, 4616-4619. (open access)
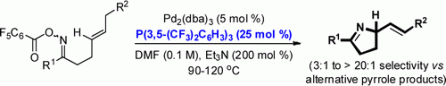
34. M. H. Shaw, E. Y. Melikhova, D. P. Kloer, W. G. Whittingham and J. F. Bower*; Directing group enhanced carbonylative ring expansions of amino-substituted cyclopropanes: rhodium catalyzed multicomponent synthesis of N-heterobicyclic enones. J. Am. Chem. Soc. 2013, 135, 4992-4995. (open access)
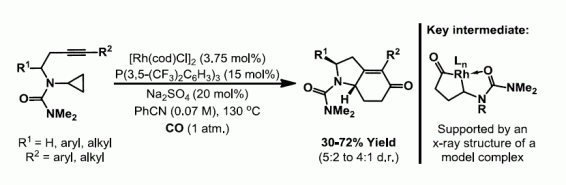
33. P. M. Murray,* J. F. Bower, D. K. Cox, E. K. Galbraith, J. S. Parker and J. B. Sweeney*; A robust first-pass protocol for the Heck-Mizoroki reaction. Org. Process Res. Dev. 2013, 17, 397-405.
32. A. Faulkner, J. S. Scott and J. F. Bower*; Palladium catalyzed cyclizations of oxime esters with 1,1-disubstituted alkenes: synthesis of α,α-disubstituted dihydropyrroles and studies towards an asymmetric protocol. Chem. Commun. 2013, 49, 1521-1523. (open access)
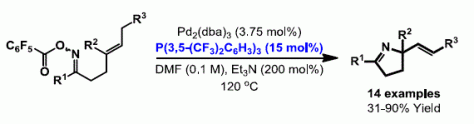
31. A. Faulkner and J. F. Bower*; Highly efficient Narasaka-Heck cyclizations mediated by P(3,5-(CF3)2C6H3)3: facile access to N-heterobicyclic scaffolds. Angew. Chem. 2012, 124, 1707-1711; Angew. Chem. Int. Ed. 2012, 51, 1675-1679.
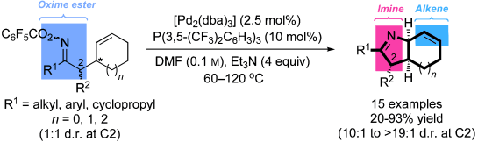
30. T. J. Donohoe,* J. F. Bower and L. K. M. Chan; Olefin cross-metathesis for the synthesis of heteroaromatic compounds. Org. Biomol. Chem. 2012, 10, 1322-1328.
-
-
-
- Highlighted as a “Hot” Emerging Area.
29. T. J. Donohoe,* J. F. Bower, D. B. Baker, J. A. Basutto, L. K. M. Chan and P. Gallagher; Synthesis of 2,4,6-trisubstituted pyridines via an olefin cross-metathesis/Heck-cyclisation-elimination sequence. Chem. Commun. 2011, 47, 10611-10613.
28. T. J. Donohoe,* J. A. Basutto, J. F. Bower and A. Rathi; Heteroaromatic synthesis via olefin cross-metathesis: entry to polysubstituted pyridines. Org. Lett. 2011, 13, 1036-1039.
27. J. F. Bower and M. J. Krische*; Formation of C-C bonds via iridium catalyzed hydrogenation and transfer hydrogenation. Top. Organomet. Chem. 2011, 34, 107-138.
26. T. J. Donohoe,* J. F. Bower and J. A. Basutto; Olefin cross-metathesis based approaches to furans: procedures for the preparation of di- and trisubstituted variants. Nature Protocols 2010, 5, 2005-2010.
25. T. J. Donohoe,* N. J. Race, J. F. Bower and C. K. A. Callens; Substituted pyrroles via olefin cross-metathesis. Org. Lett. 2010, 12, 4094-4097.
24. T. J. Donohoe* and J. F. Bower; An expedient route to substituted furans via olefin cross-metathesis. Proc. Natl. Acad. Sci. U.S.A. 2010,107, 3373-3376.
23. J. F. Bower,* J. Rujirawanich and T. Gallagher*; N-Heterocycle construction via cyclic sulfamidates. Applications in synthesis. Org. Biomol. Chem. 2010, 8, 1505-1519.
22. T. J. Donohoe,* J. F. Bower, J. A. Basutto, L. P. Fishlock, P. A. Procopiou and C. K. A. Callens; Ring-closing metathesis for the synthesis of heteroaromatics: evaluating routes to pyridines and pyridazines. Tetrahedron 2009, 65, Symposium-in-print, Modern applications of transition metal catalysis in heterocycle synthesis, 8969-8980.
21. T. J. Donohoe,* L. P. Fishlock, J. A. Basutto, J. F. Bower, P. A. Procopiou and A. L. Thompson; Synthesis of substituted pyridines and pyridazines via ring closing metathesis. Chem. Commun. 2009, 3008-3010.
20. J. F. Bower and M. J. Krische*; Hydrogenation for C-C bond formation. In Handbook of Green Chemistry – Green Catalysis, Volume 1: Homogeneous Catalysis. P. T. Anastas and R. H. Crabtree (Eds.), Wiley-VCH: Weinheim, 2009, 205-254.
19. J. F. Bower, I. S. Kim, R. L. Patman and M. J. Krische*; Catalytic carbonyl addition through transfer hydrogenation: a departure from preformed organometallic reagents. Angew. Chem. 2009, 121, 36-48; Angew. Chem. Int. Ed. 2009, 48, 34-46.
18. R. L. Patman, J. F. Bower, I. S. Kim and M. J. Krische*; Formation of C-C bonds via catalytic hydrogenation and transfer hydrogenation: vinylation, allylation and enolate addition of carbonyl compounds and imines. Aldrichimica Acta 2008, 41, 95-104.
17. J. S. Parker,* J. F. Bower, P. M. Murray, B. Patel and P. Talavera; Kepner-Tregoe decision analysis as a tool to aid route selection. Part 3. Application to a back-up series of compounds in the PDK project. Org. Process Res. Dev. 2008, 12, 1060-1077.
16. F. Shibahara, J. F. Bower and M. J. Krische*; Diene hydroacylation from the alcohol or aldehyde oxidation level via ruthenium-catalyzed C-C bond-forming transfer hydrogenation: synthesis of β,γ-unsaturated ketones. J. Am. Chem. Soc. 2008, 130, 14120-14122.
15. R. L. Patman, V. M. Williams, J. F. Bower and M. J. Krische*; Carbonyl propargylation from the alcohol or aldehyde oxidation level employing 1,3-enynes as surrogates to preformed allenyl metal reagents: a ruthenium catalyzed C-C bond forming transfer hydrogenation. Angew. Chem. 2008, 120, 5298-5301; Angew. Chem. Int. Ed. 2008, 47, 5220-5223.
14. F. Shibahara, J. F. Bower and M. J. Krische*; Ruthenium catalyzed C-C bond forming transfer hydrogenation: carbonyl allylation from the alcohol or aldehyde oxidation level employing acyclic 1,3-dienes as surrogates to preformed allyl metal reagents. J. Am. Chem. Soc. 2008, 130, 6338-6339.
13. J. F. Bower, R. L. Patman and M. J. Krische*; Iridium catalyzed C-C coupling via transfer hydrogenation: carbonyl addition from the alcohol or aldehyde oxidation level employing 1,3-cyclohexadiene. Org. Lett. 2008, 10, 1033-1035.
-
-
-
- Highlighted in SYNFACTS 2008, 505.
12. J. F. Bower, E. Skucas, R. L. Patman and M. J. Krische*; Catalytic C-C coupling via transfer hydrogenation: reverse prenylation, crotylation and allylation from the alcohol or aldehyde oxidation level. J. Am. Chem. Soc. 2007, 129, 15134-15135.
-
-
-
- Highlighted in SYNFACTS 2008, 293.
11. E. Skucas, J. F. Bower and M. J. Krische*; Carbonyl allylation in the absence of preformed allyl metal reagents: reverse prenylation via iridium catalysed hydrogenative coupling of dimethylallene. J. Am. Chem. Soc. 2007, 129, 12678-12679.
10. J. F. Bower, P. Szeto and T. Gallagher*; Cyclic sulfamidates as precursors to alkylidene pyrrolidines and piperidines. Org. Lett. 2007, 9, 4909-4912.
9. J. F. Bower, P. Szeto and T. Gallagher*; Enantiopure 1,4-benzoxazines via 1,2-cyclic sulfamidates. Synthesis of levofloxacin. Org. Lett. 2007, 9, 3283-3286.
8. J. F. Bower, A. J. Williams, H. Woodward, P. Szeto, R. M. Lawrence and T. Gallagher*; Reactivity of cyclic sulfamidates towards phosphonate-stabilised enolates: synthesis and applications of α-phosphono lactams. Org. Biomol. Chem. 2007, 5, 2636-2644.
7. J. F. Bower, T. Riis-Johannessen, P. Szeto, A. J. Whitehead and T. Gallagher*; Stereospecific construction of substituted piperidines. Synthesis of (-)-paroxetine and (+)-laccarin. Chem. Commun. 2007,728-730.
-
-
-
- Highlighted in SYNFACTS 2007, 574.
6. J. F. Bower, P. Szeto and T. Gallagher*; Cyclic sulfamidates as versatile lactam precursors. An evaluation of synthetic strategies towards (-)-aphanorphine. Org. Biomol. Chem. 2007, 5, 143-150.
5. J. F. Bower, S. Chakthong, J. Švenda, A. J. Williams, R. M. Lawrence, P. Szeto and T. Gallagher*; Reactivity of cyclic sulfamidates towards sulfur-stabilised enolates. Stereocontrolled synthesis of functionalised lactams. Org. Biomol. Chem. 2006, 4, 1868-1877.
4. J. F. Bower, P. Szeto and T. Gallagher*; Cyclic sulfamidates as lactam precursors. An efficient asymmetric synthesis of (-)-aphanorphine. Chem. Commun. 2005, 5793-5795.
3. J. F. Bower, J. Švenda, A. J. Williams, J. P. H. Charmant, R. M. Lawrence, P. Szeto and T. Gallagher*; Cyclic sulfamidates as vehicles for the synthesis of substituted lactams. Org. Lett. 2004, 6, 4727-4730.
2. J. F. Bower, S. A. Cotton,* J. Fawcett, R. S. Hughes and D. R. Russell; Praseodymium complexes of 2,2′-bipyridine; the crystal and molecular structures of Pr(bipy)3(NCS)3, Pr(bipy)2(NO3)3, Pr(bipy)2Cl3(OH2)·EtOH and Pr(bipy)(S2CNEt2)3. Polyhedron 2003, 22, 347-354.
1. J. F. Bower, S. A. Cotton,* J. Fawcett* and D. R. Russell; Bis(2,2′-bipyridyl-N,N‘)tris(nitrato-O,O‘)neodymium. Acta Cryst. 2000, C56, e8-e9.